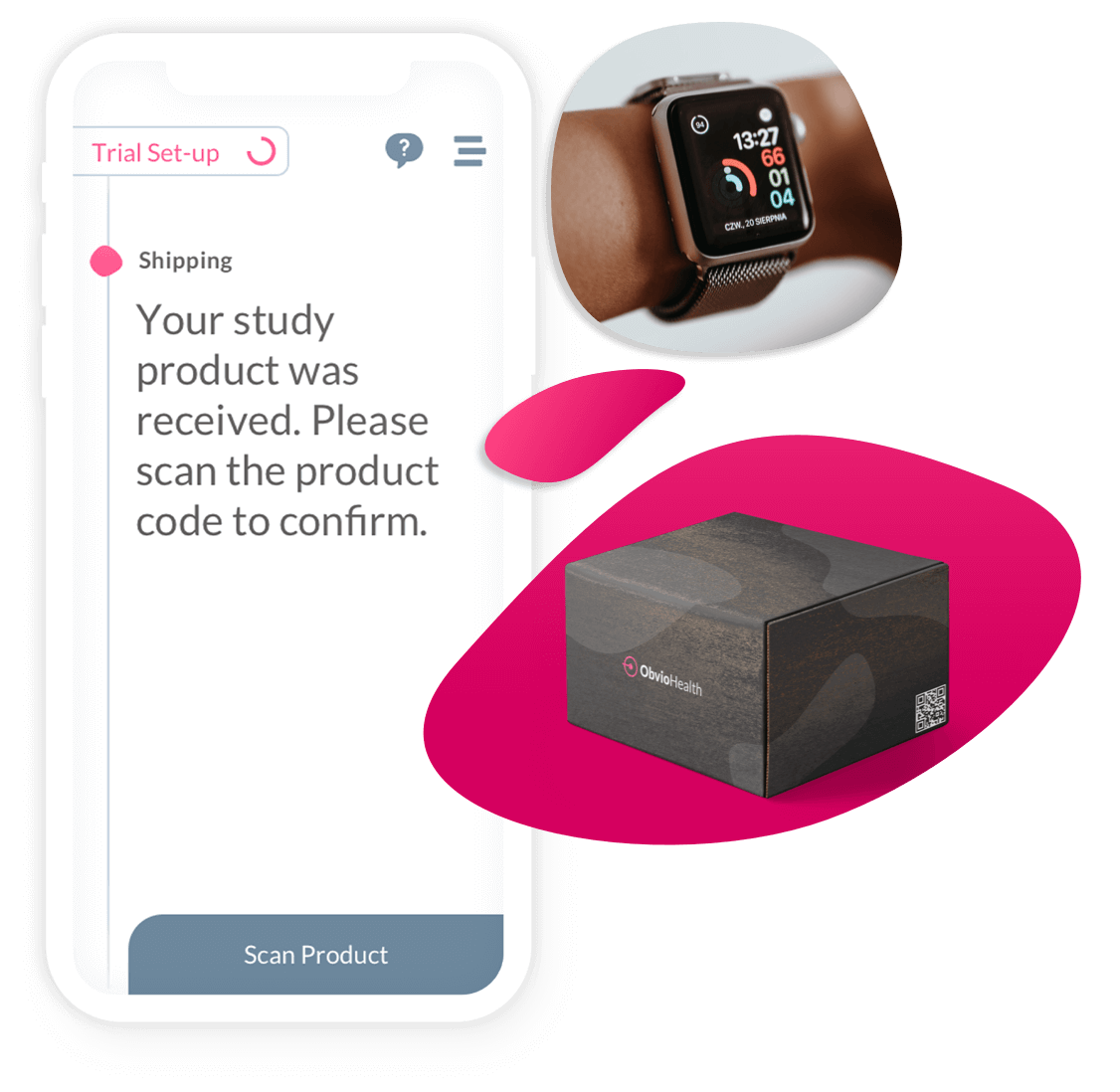
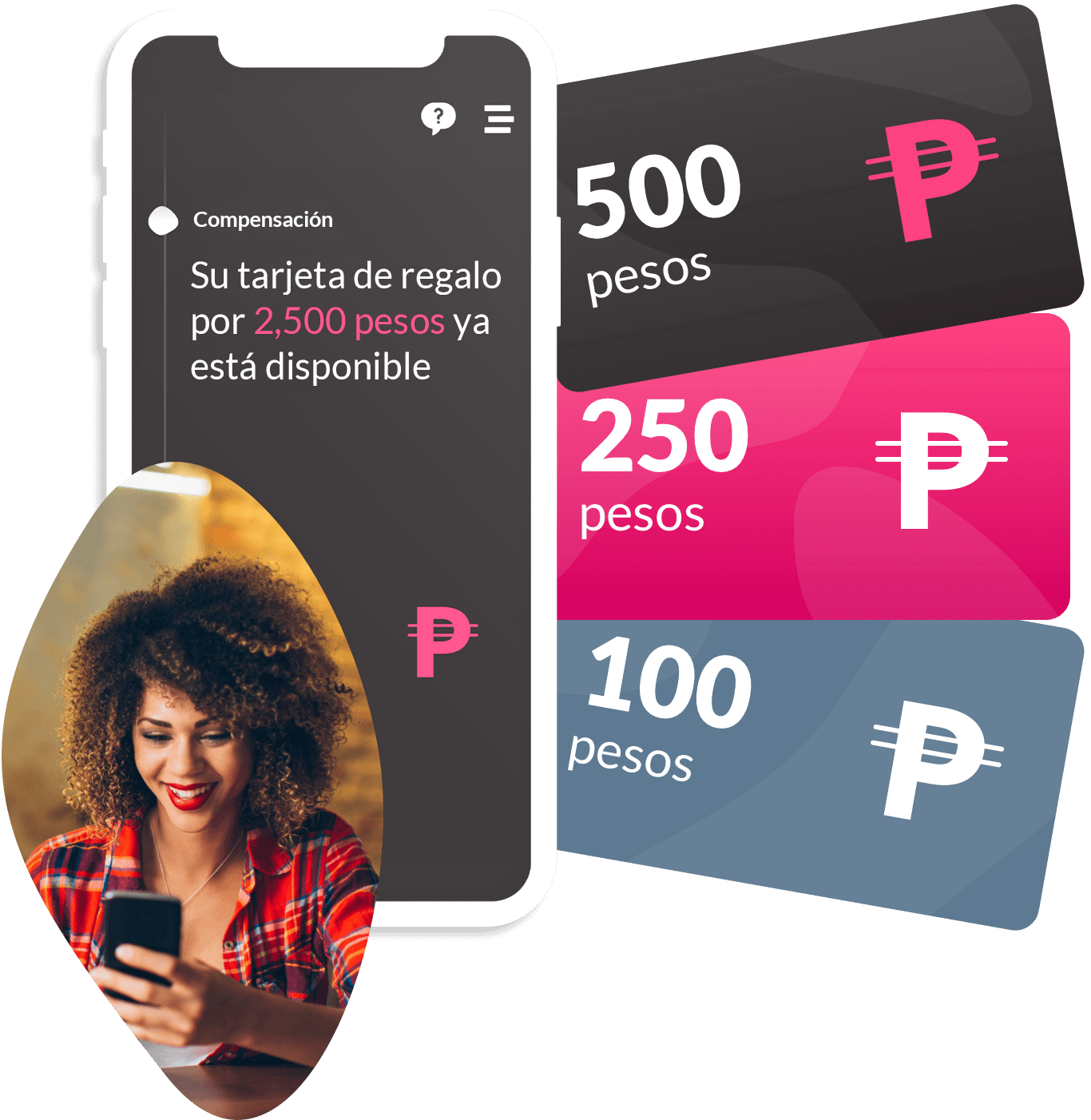
Step 4:
Logistics & Device Management
Connected. Configurable. Built for Scale.
Clinical trial logistics shouldn’t be a barrier to speed or scale. ObvioHealth delivers an integrated logistics and device management solution—designed specifically for decentralized and hybrid clinical trials. From direct-to-patient shipments to digital reimbursements, our platform ensures that every moving part of your trial is delivered with precision, compliance, and visibility.