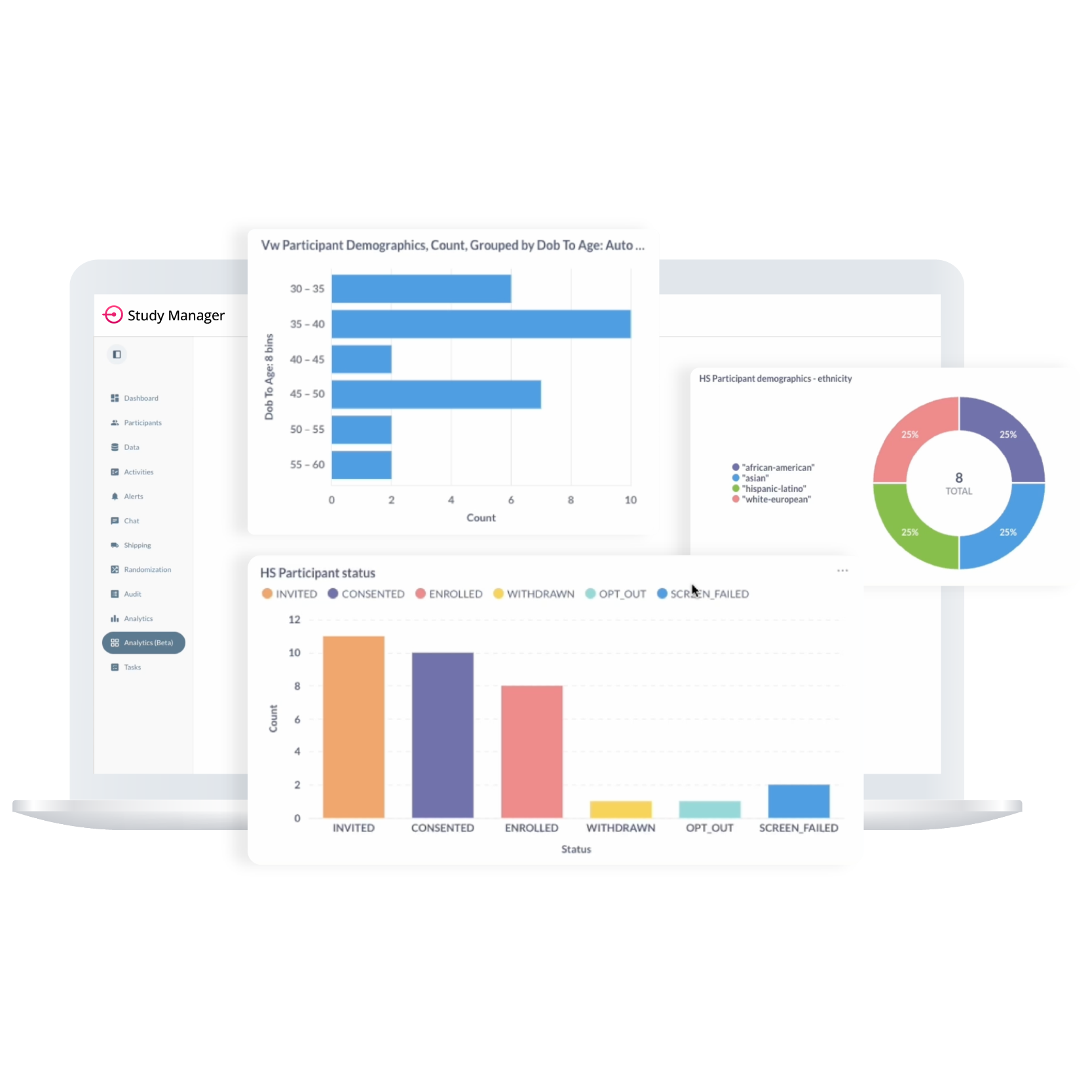
Step 7:
Analysis & Reporting
From Raw Data to Real-Time Insight—
All in One Platform
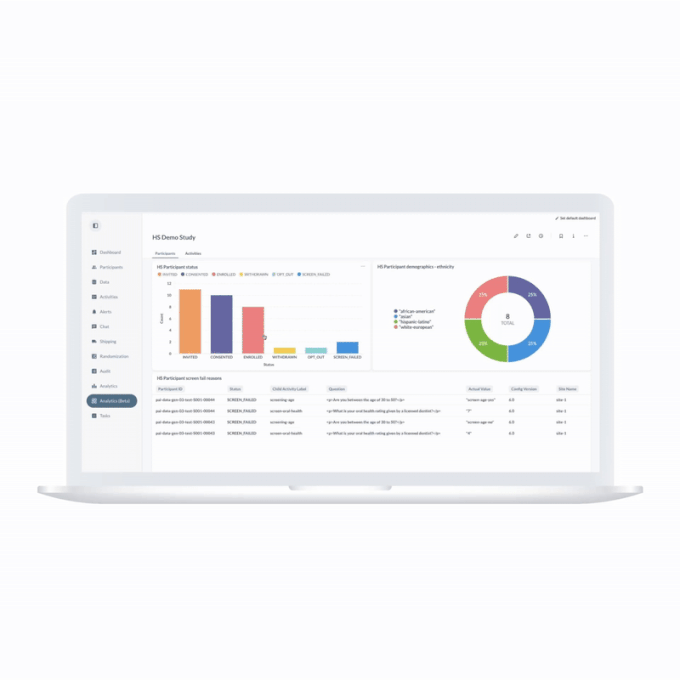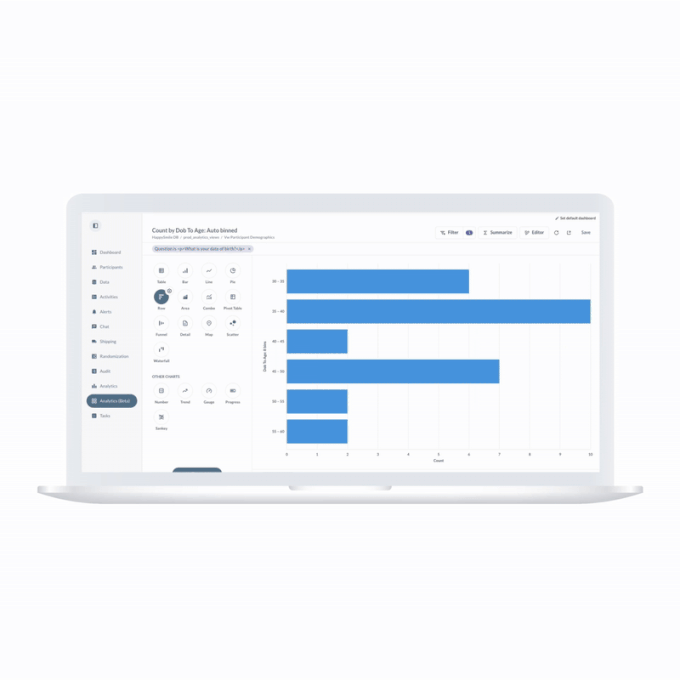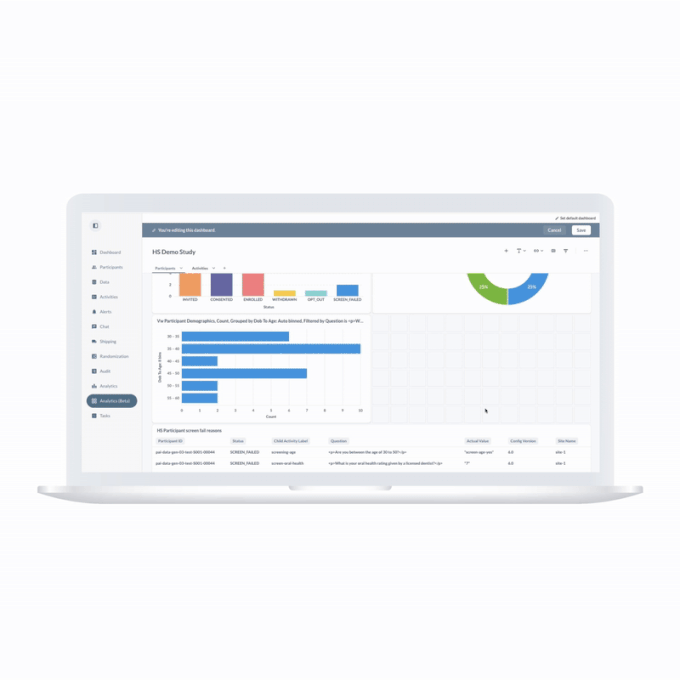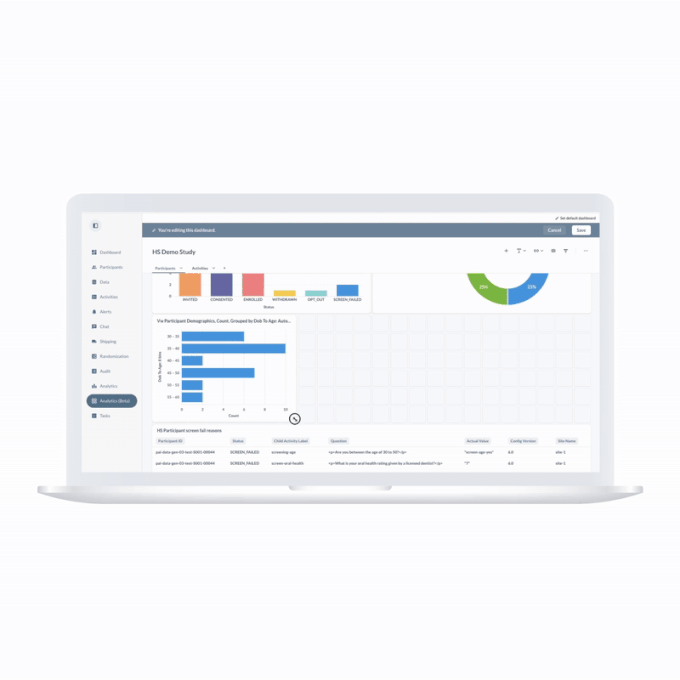
ObvioGo transforms decentralized trial data into actionable insight. With built-in analytics and AI-powered reporting tools, study teams can monitor progress, spot deviations, and make smarter decisions—faster.