Step 6:
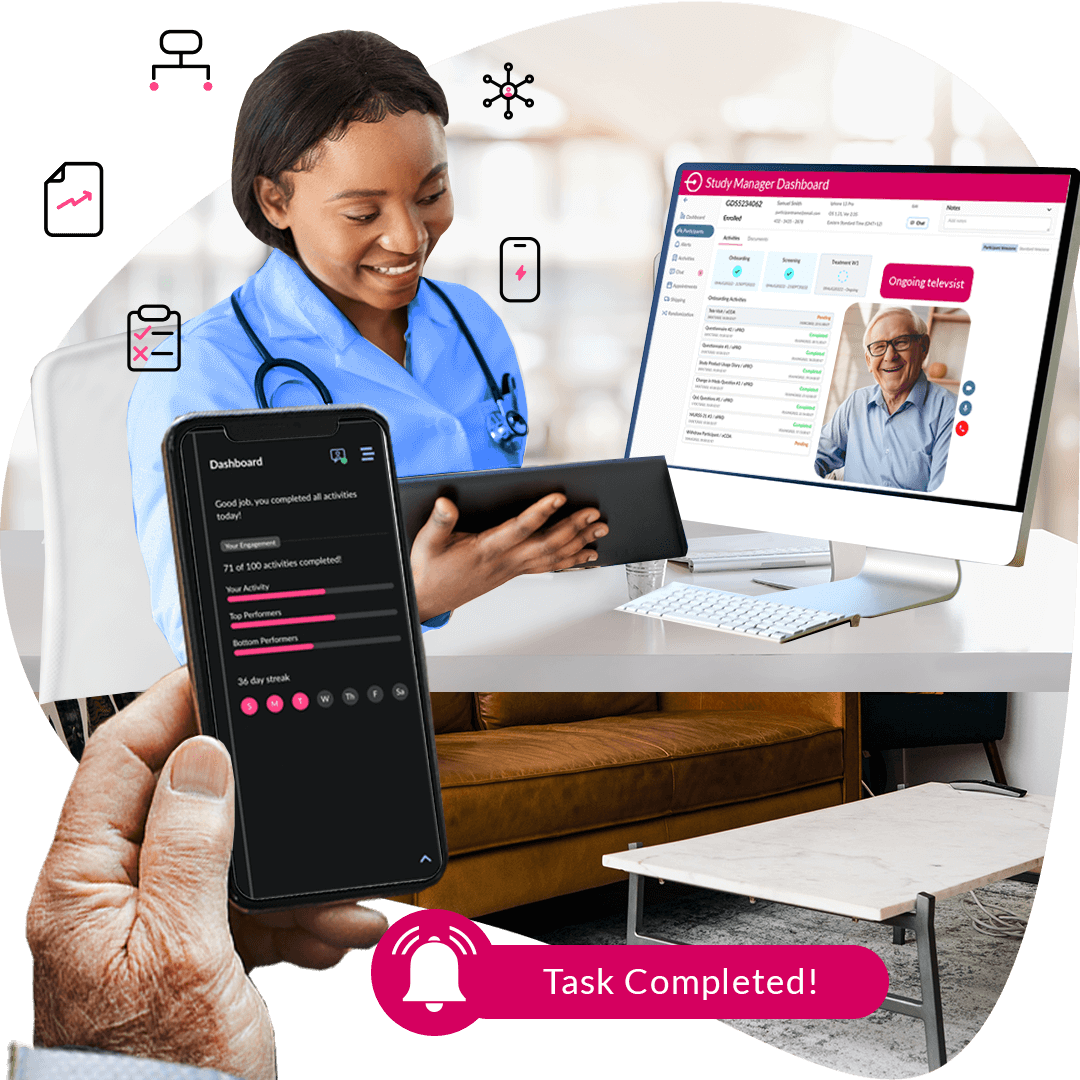
Virtual Visits & Support
Bringing real-time support and trial visits directly to patients—anytime, anywhere.
ObvioGo enables secure, real-time interactions between study teams and participants—reducing the need for in-person visits while maintaining compliance and protocol fidelity. By supporting decentralized interactions, we help sponsors deliver flexible, scalable trials with improved retention and data accuracy.