
An ultra-flexible clinical trials platform that delivers better data, faster.

ObvioGo is an all-in-one, no-code platform that unifies study design, oversight, and participant engagement in a single, intuitive system.
Built for sponsors, CROs, and digital health innovators, it streamlines configuration, shortens startup timelines, and improves compliance and data quality—empowering teams to launch and scale trials without developers or IT support.
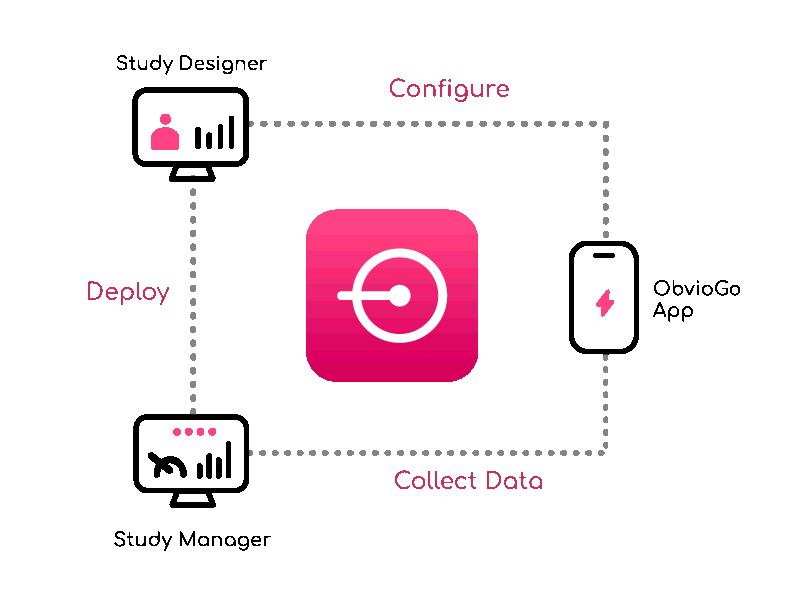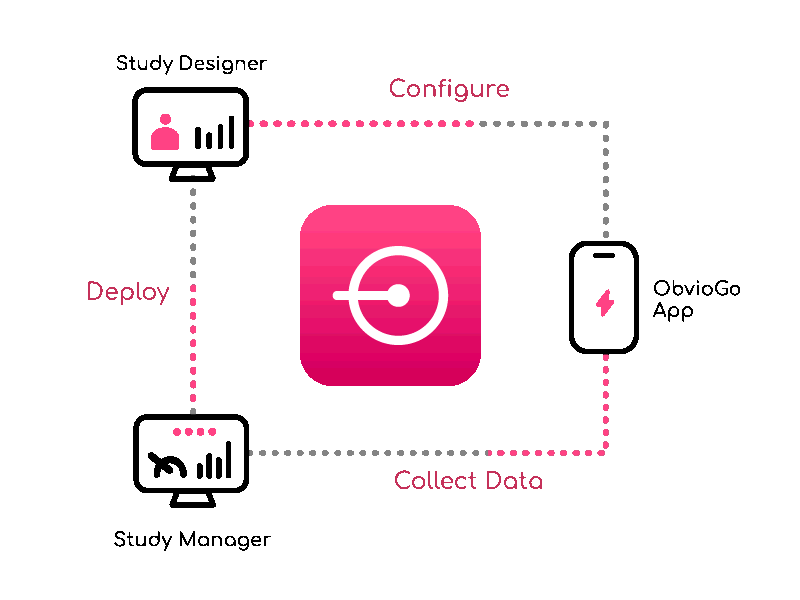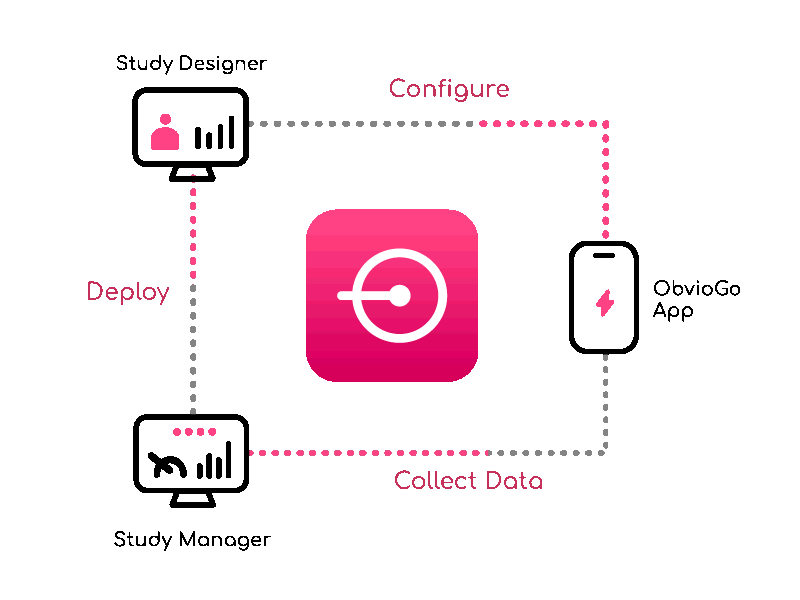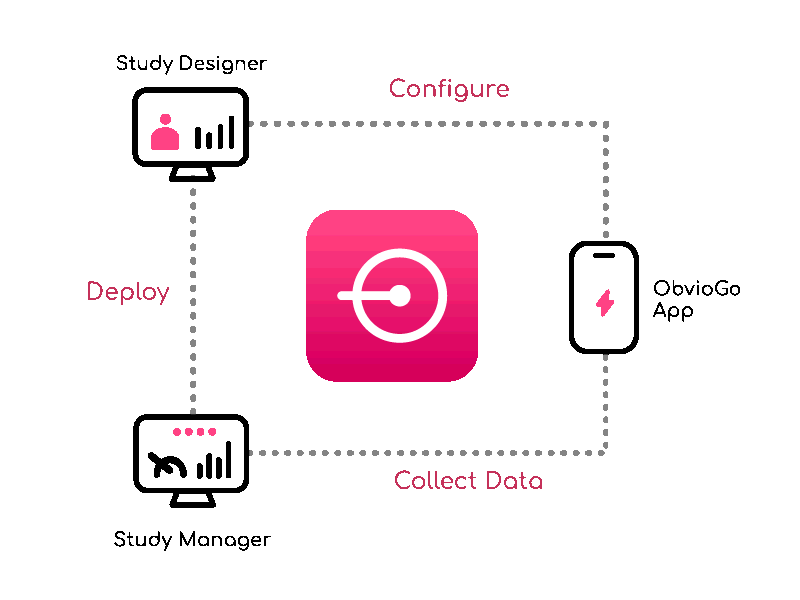











Build study protocols quick with Study Designer — visually configure workflows, data, and branching.
.gif)

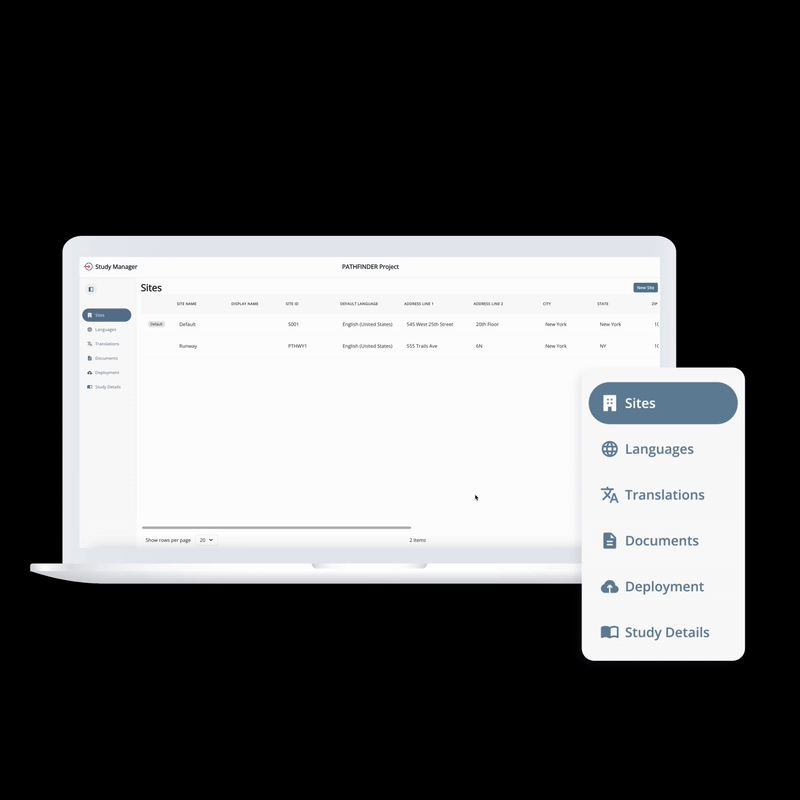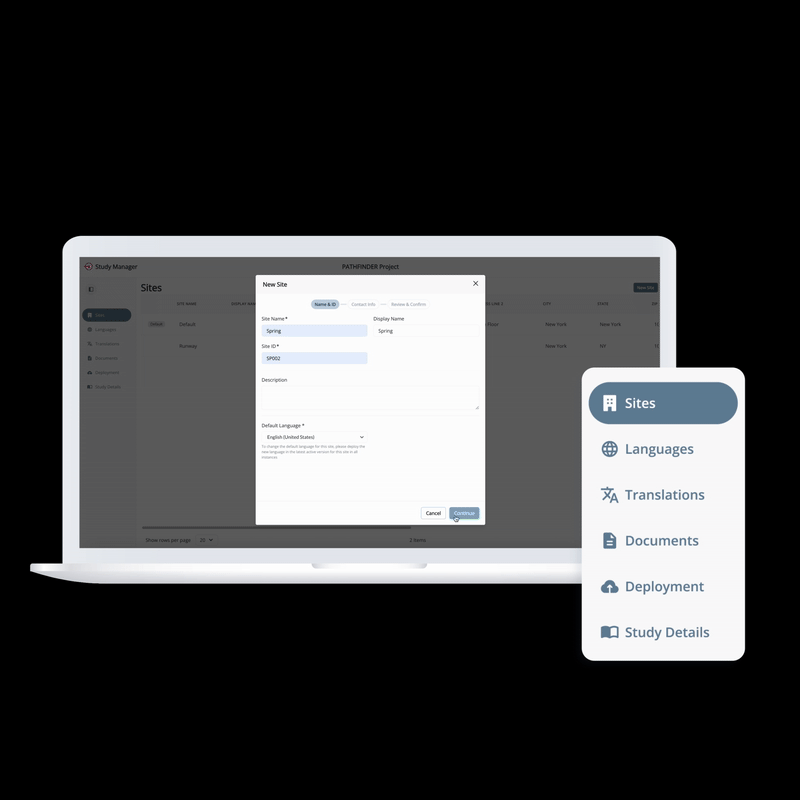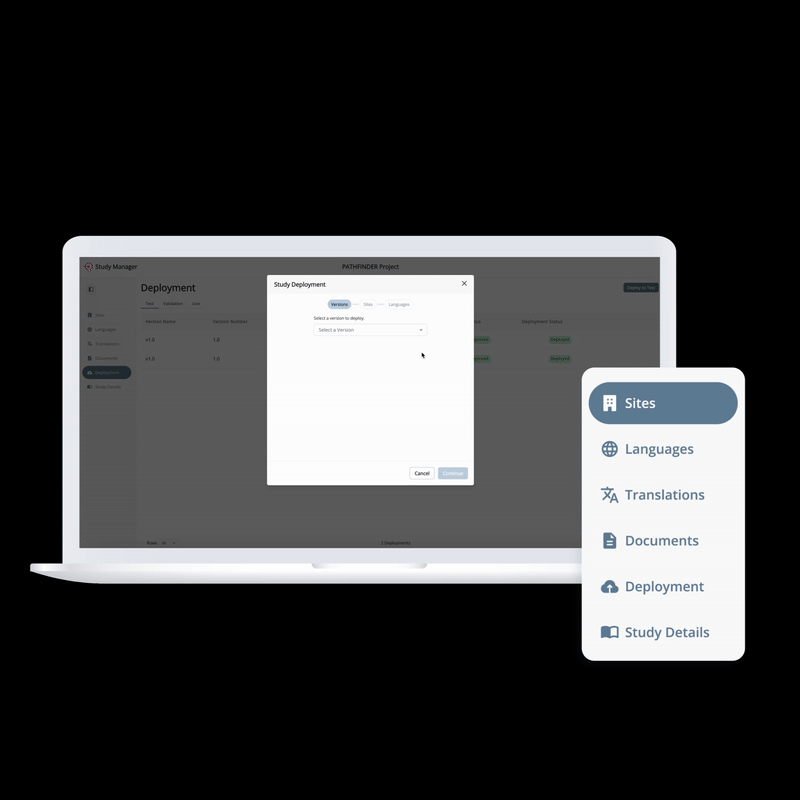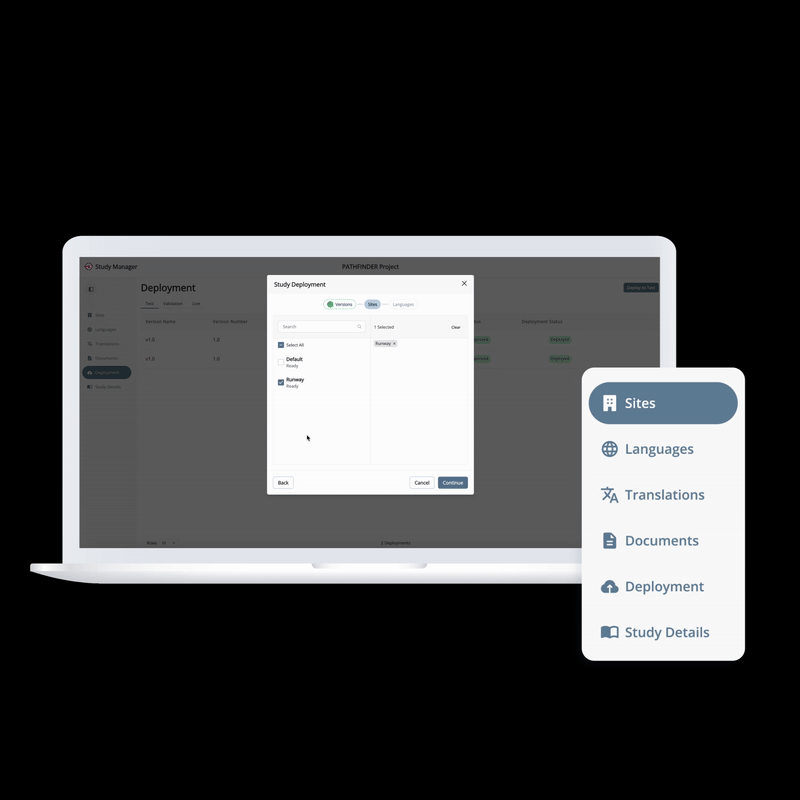
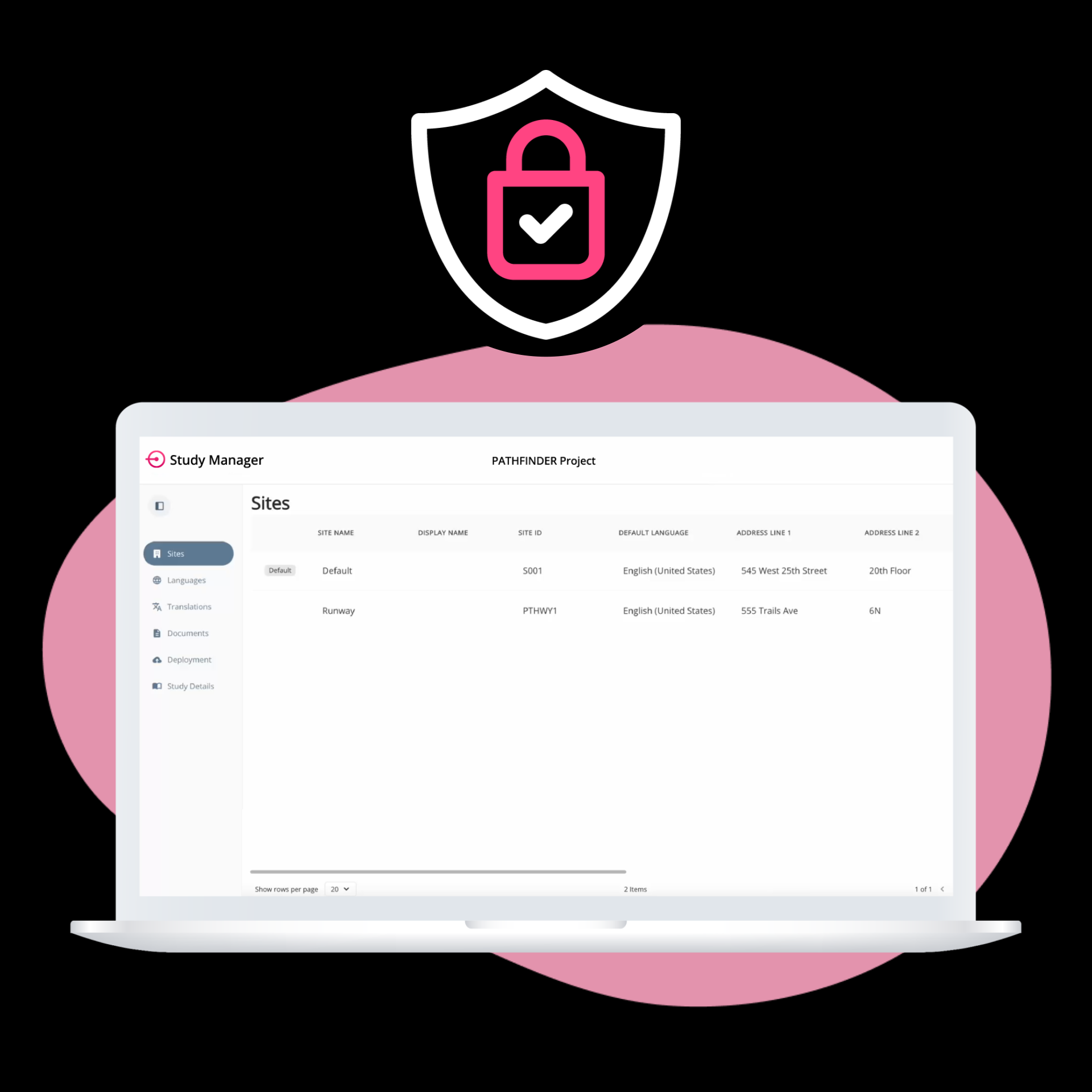
From setup to execution, Study Manager simplifies and centralizes your trial operations.

ObvioGo brings trials to participants with an intuitive app that drives engagement, compliance, and connection.
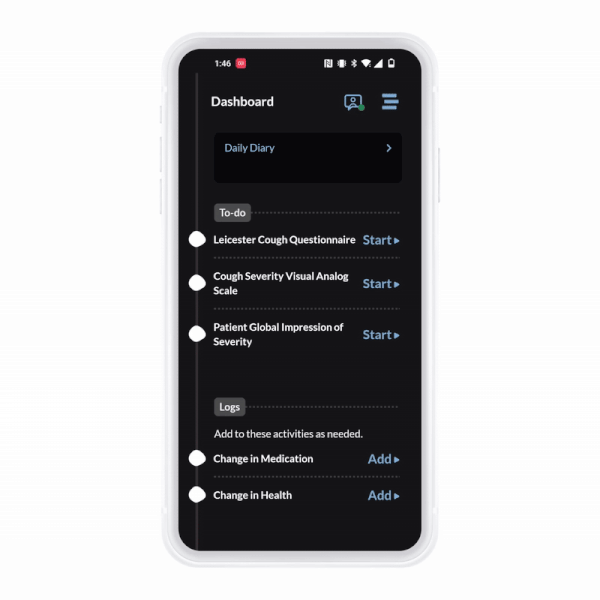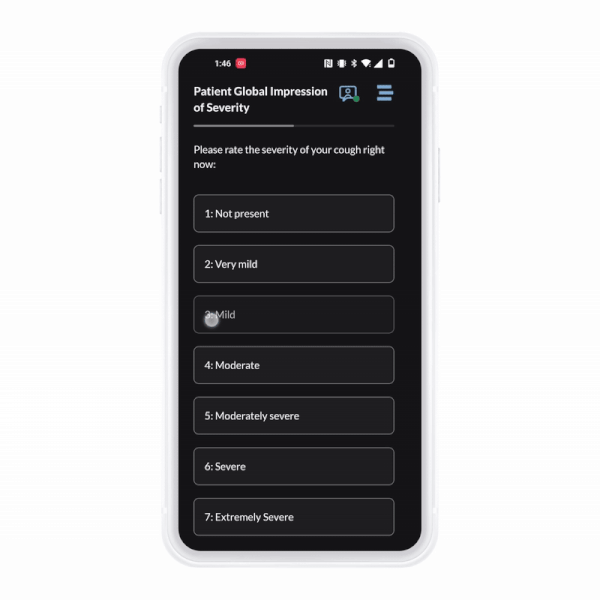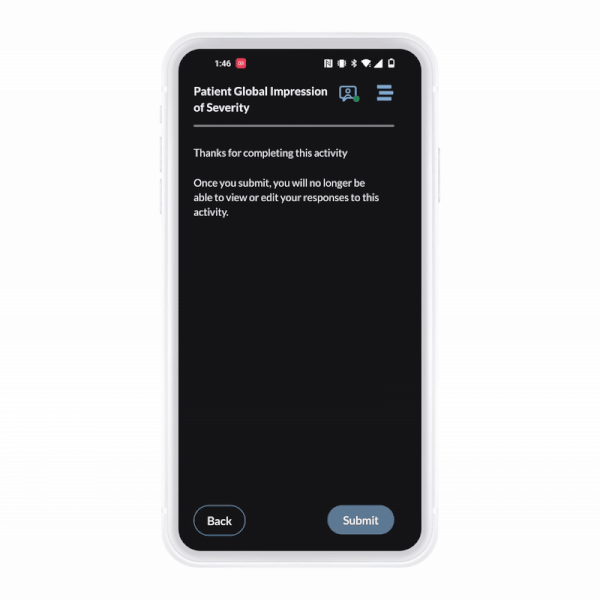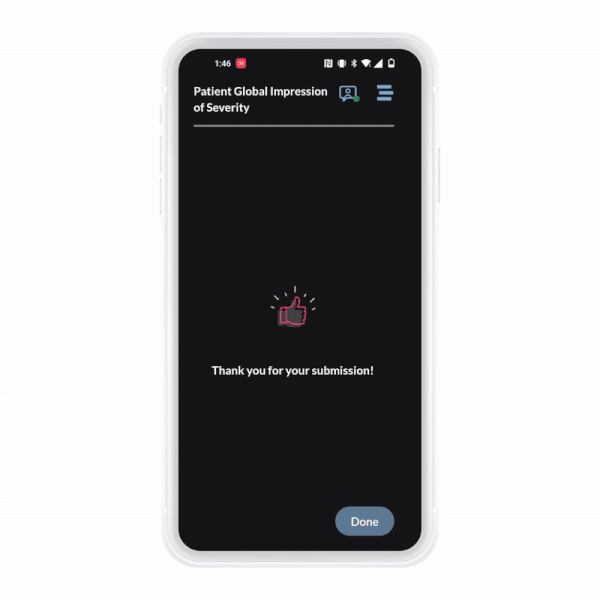

ObvioGo makes trials inspection-ready with built-in compliance and security.

ObvioGo connects seamlessly across your tech stack, powering efficient, scalable trials.


“We knew we needed to make the study easy for our patients. We had the added stress of launching this trial at the height of the pandemic. ObvioHealth's virtual site delivered the support we needed for our patients, and their clinical science team provided expertise without the rigidity of a large CRO.”
